-

31-12-2019 |
Kautilya Academy
-

31-10-2020 |
Kautilya Academy
-

31-08-2020 |
Kautilya Academy
-

31-07-2019 |
KAUTILYA ACADEMY
-

31-03-2020 |
Kautilya Academy
-

31-01-2023 |
Kautilya Academy
-

31-01-2019 |
Kautilya Academy
-

30-11-2019 |
Kautilya Academy
-

30-11-2019 |
Kautilya Academy
-
30-09-2020 |
Kautilya Academy
-

30-07-2020 |
Kautilya Academy
-

30-07-2019 |
Kautilya Academy
-

30-06-2020 |
Kautilya Academy
-
30-06-2020 |
Kautilya Academy
-

30-05-2020 |
Kautilya Academy
-

30-04-2020 |
Kautilya Academy
-
30-03-2020 |
Kautilya Academy
-

30-01-2019 |
Kautilya academy
-
.jpg)
30-01-2019 |
Kautilya academy
-
.jpg)
30-01-2019 |
Kautilya academy
-

30-01-2019 |
Kautilya Academy
-

29-11-2023 |
Kautilya Academy
-

29-11-2019 |
Kautilya Academy
-

29-10-2020 |
Kautilya Academy
-

29-09-2020 |
Kautilya Academy
-

29-08-2020 |
Kautilya Academy
-

29-05-2020 |
Kautilya Academy
-

29-04-2020 |
-

29-04-2019 |
Kautilya Academy
-

29-01-2020 |
Kautilya Academy
-

28-11-2019 |
Kautilya Academy
-

28-10-2020 |
Kautilya Academy
-

28-09-2020 |
-

28-08-2020 |
Kautilya Academy
-

28-07-2020 |
Kautilya Academy
-
28-05-2020 |
Kautilya Academy
-

28-04-2020 |
Kautilya Academy
-

28-03-2020 |
Kautilya Academy
-

28-02-2020 |
Kautilya Academy
-

28-01-2019 |
Kautilya Academy
-

27-12-2018 |
Kautilya Academy
-

27-11-2020 |
Kautilya Academy
-

27-11-2019 |
kautilya academy
-

27-10-2023 |
Kautilya Academy
-

27-10-2020 |
Kautilya Academy
-

27-08-2020 |
Kautilya Academy
-

27-07-2023 |
Kautilya Academy
-

27-07-2020 |
Kautilya Academy
-

27-05-2021 |
Kautilya Academy
-

27-05-2020 |
Kautilya Academy
-

27-04-2020 |
Kautilya Academy
-

27-03-2020 |
Kautilya Academy
-

27-01-2024 |
Kautilya Academy
-

26-12-2019 |
Kautilya Academy
-
26-12-2018 |
Shridhant Joshi
-

26-12-2018 |
Shridhant Joshi
-

26-11-2019 |
Kautilya Academy
-

26-11-2019 |
kautilya academy
-
.png)
26-09-2020 |
Kautilya Academy
-

26-08-2023 |
Kautilya Academy
-

26-08-2020 |
Kautilya Academy
-

26-06-2020 |
Kautilya Academy
-

26-05-2021 |
Kautilya Academy
-

26-03-2020 |
Kautilya Academy
-
26-02-2020 |
Kautilya Academy
-

25-12-2020 |
Kautilya Academy
-

25-12-2020 |
Kautilya Academy
-

25-12-2018 |
Kautilya Academy
-

25-12-2018 |
Kautilya Academy
-

25-11-2020 |
Kautilya Academy
-
25-11-2019 |
Kautilya Academy
-
25-11-2019 |
Kautilya Academy
-

25-09-2020 |
Kautilya Academy
-

25-08-2020 |
Kautilya Academy
-

25-07-2020 |
Kautilya Academy
-

25-06-2020 |
Kautilya Academy
-

25-05-2020 |
Kautilya Academy
-

25-04-2020 |
-

25-03-2020 |
Kautilya Academy
-

25-02-2020 |
Kautilya Academy
-

25-01-2020 |
Kautilya Academy
-
.jpg)
24-12-2019 |
Kautilya Academy
-

24-11-2020 |
Kautilya Academy
-

24-09-2020 |
Kautilya Academy
-

24-08-2020 |
Kautilya Academy
-

24-08-2019 |
Kautilya Academy
-

24-06-2020 |
Kautilya Academy
-

24-05-2021 |
Kautilya Academy
-

24-04-2020 |
Kautilya Academy
-

24-03-2023 |
Kautilya Academy
-

24-03-2020 |
Kautilya Academy
-

24-02-2020 |
Kautilya Academy
-
24-01-2020 |
Kautilya Academy
-

23-11-2021 |
Kautilya Academy
-

23-11-2020 |
Kautilya Academy
-

23-11-2019 |
Kautilya Academy
-

23-11-2019 |
Kautilya Academy
-

23-11-2019 |
Kautilya Academy
-

23-09-2020 |
Kautilya Academy
-
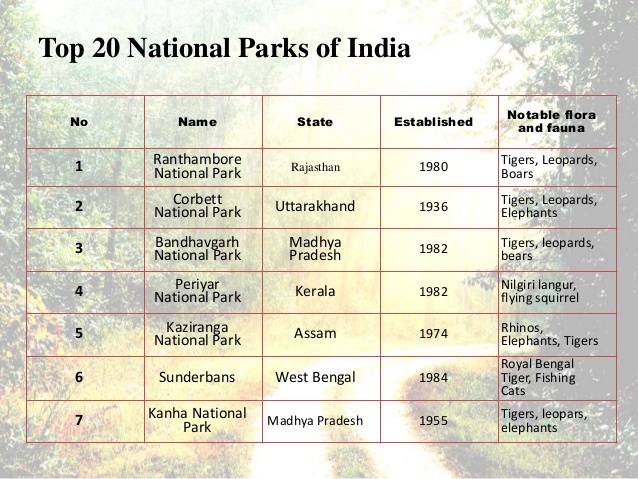
23-09-2019 |
Kautilya Academy
-

23-09-2019 |
Kautilya Academy
-

23-09-2019 |
Kautilya Academy
-

23-09-2019 |
Kautilya Academy
-

23-09-2019 |
Kautilya Academy
-

23-07-2021 |
Kautilya Academy
-

23-07-2020 |
Kautilya Academy
-

23-06-2020 |
Kautilya Academy
-

23-05-2020 |
Kautilya Academy
-

23-04-2020 |
Kautilya Academy
-

23-03-2020 |
Kautilya Academy
-

23-01-2020 |
Kautilya Academy
-
.jpg)
22-11-2023 |
Kautilya Academy
-
.jpg)
22-11-2019 |
Kautilya Academy
-
.jpg)
22-11-2019 |
Kautilya Academy
-
.jpg)
22-11-2019 |
Kautilya Academy
-

22-10-2020 |
Kautilya Academy
-

22-09-2023 |
Kautilya Academy
-

22-09-2020 |
Kautilya Academy
-

22-08-2020 |
Kautilya Academy
-

22-07-2023 |
Kautilya Academy
-

22-06-2020 |
Kautilya Academy
-

22-05-2021 |
Kautilya Academy
-

22-05-2020 |
Kautilya Academy
-

22-04-2020 |
Kautilya Academy
-

22-02-2021 |
Kautilya Academy
-

22-01-2020 |
Kautilya Academy
-

21-12-2023 |
Kautilya Academy
-

21-11-2020 |
Kautilya Academy
-

21-11-2019 |
Kautilya Academy
-

21-11-2019 |
Kautilya Academy
-

21-11-2019 |
Kautilya Academy
-
21-11-2019 |
Kautilya Academy
-

21-10-2020 |
Kautilya Academy
-

21-10-2019 |
Kautilya Academy
-

21-10-2019 |
Kautilya Academy
-

21-09-2020 |
Kautilya Academy
-

21-09-2020 |
Kautilya Academy
-

21-08-2020 |
Kautilya Academy
-

21-08-2020 |
Kautilya Academy
-

21-08-2020 |
Kautilya Academy
-

21-08-2019 |
Kautilya academy
-

21-07-2020 |
Kautilya Academy
-

21-05-2021 |
Kautilya Academy
-

21-05-2020 |
Kautilya Academy
-

21-04-2023 |
Kautilya Academy
-
21-04-2020 |
Kautilya Academy
-

21-03-2023 |
Kautilya Academy
-

21-03-2023 |
Kautilya Academy
-

21-01-2020 |
Kautilya Academy
-

20-11-2019 |
Kautilya Academy
-

20-11-2019 |
Kautilya Academy
-

20-11-2019 |
Kautilya Academy
-

20-10-2020 |
Kautilya Academy
-

20-09-2019 |
Kautilya Academy
-
20-07-2020 |
-
20-06-2020 |
Kautilya Academy
-

20-05-2021 |
Kautilya Academy
-

20-05-2020 |
Vishnugupta Academy
-

20-04-2023 |
Kautilya Academy
-

20-04-2020 |
Kautilya Academy
-

20-02-2020 |
Kautilya Academy
-

20-01-2020 |
Kautilya Academy
-

19-12-2020 |
Kautilya Academy
-

19-11-2019 |
Kautilya Academy
-

19-10-2023 |
Kautilya Academy
-

19-10-2020 |
Kautilya Academy
-

19-09-2019 |
Kautilya Academy
-

19-09-2019 |
Kautilya Academy
-

19-09-2019 |
Kautilya Academy
-

19-09-2019 |
Kautilya Academy
-

19-09-2019 |
Kautilya Academy
-

19-07-2023 |
Kautilya Academy
-

19-06-2020 |
Kautilya Academy
-

19-05-2021 |
Kautilya Academy
-

19-05-2020 |
Kautilya Academy
-

19-03-2020 |
Kautilya Academy
-

19-01-2019 |
Kautilya Academy
-
.jpg)
19-01-2019 |
Kautilya Academy
-

18-12-2023 |
Kautilya Academy
-

18-11-2019 |
Kautilya Academy
-

18-11-2019 |
Kautilya Academy
-

18-11-2019 |
Kautilya Academy
-

18-11-2019 |
Kautilya Academy
-

18-11-2019 |
Kautilya Academy
-

18-11-2019 |
Kautilya Academy
-

18-09-2020 |
Kautilya Academy
-

18-07-2020 |
Kautilya Academy
-

18-06-2020 |
Kautilya Academy
-

18-05-2020 |
-

18-04-2020 |
Kautilya Academy
-

18-03-2020 |
Kautilya Academy
-

18-01-2023 |
Kautilya Academy
-

18-01-2020 |
Kautilya Academy
-

17-09-2020 |
Kautilya Academy
-

17-09-2019 |
Kautilya Academy
-

17-08-2023 |
Kautilya Academy
-

17-07-2020 |
Kautilya Academy
-

17-06-2020 |
Kautilya Academy
-

17-05-2021 |
Kautilya Academy
-

17-04-2020 |
Kautilya Academy
-

17-02-2020 |
Kautilya Academy
-

17-01-2020 |
Kautilya Academy
-
1.jpg)
16-12-2020 |
Kautilya Academy
-

16-12-2019 |
Kautilya Academy
-
16-11-2019 |
Kautilya Academy
-

16-11-2019 |
Kautilya Academy
-

16-11-2019 |
Kautilya Academy
-

16-11-2019 |
Kautilya Academy
-

16-11-2019 |
Kautilya Academy
-

16-11-2019 |
Kautilya Academy
-

16-11-2019 |
Kautilya Academy
-

16-11-2019 |
Kautilya Academy
-

16-09-2020 |
Kautilya Academy
-

16-09-2019 |
Kautilya Academy
-

16-07-2020 |
Kautilya Academy
-

16-06-2022 |
Kautilya Academy
-
16-06-2020 |
Kautilya Academy
-
16-05-2020 |
Kautilya Academy
-

16-04-2020 |
Kautilya Academy
-

16-03-2020 |
Kautilya Academy
-

16-01-2020 |
Kautilya Academy
-

16-01-2019 |
Kautilya Academy
-

15-12-2020 |
Kautilya Academy
-

15-11-2019 |
Kautilya Academy
-
.jpg)
15-11-2019 |
Kautilya Academy
-

15-10-2020 |
Kautilya Academy
-

15-09-2020 |
Kautilya Academy
-

15-07-2020 |
Kautilya Academy
-

15-06-2020 |
Kautilya Academy
-

15-05-2021 |
Kautilya Academy
-

15-05-2020 |
Kautilya Academy
-

15-04-2020 |
Kautilya Academy
-

15-03-2023 |
Kautilya Academy
-
15-02-2020 |
Kautilya Academy
-

15-01-2021 |
Kautilya Academy
-

14-12-2020 |
Kautilya Academy
-

14-12-2019 |
Kautilya Academy
-

14-10-2020 |
Kautilya Academy
-
.jpg)
14-09-2023 |
Kautilya Academy
-

14-09-2020 |
Kautilya Academy
-

14-09-2019 |
Kautilya Academy
-

14-07-2023 |
Kautilya Academy
-
.jpg)
14-07-2020 |
-

14-05-2020 |
Kautilya Academy
-

14-04-2020 |
Kautilya Academy
-

13-12-2019 |
Kautilya Academy
-

13-11-2019 |
Kautilya Academy
-
1.jpg)
13-10-2022 |
Kautilya Academy
-

13-10-2020 |
Kautilya Academy
-

13-07-2020 |
Kautilya Academy
-

13-05-2020 |
Kautilya Academy
-
13-04-2020 |
Kautilya Academy
-
.jpg)
13-02-2019 |
Kautilya Academy
-

12-12-2019 |
Kautilya Academy
-

12-11-2019 |
Kautilya Academy
-

12-11-2019 |
Kautilya Academy
-

12-11-2019 |
Kautilya Academy
-

12-11-2019 |
Kautilya Academy
-

12-11-2019 |
Kautilya Academy
-

12-10-2023 |
Kautilya Academy
-

12-09-2020 |
Kautilya Academy
-

12-08-2023 |
Kautilya Academy
-

12-08-2019 |
Kautilya Academy
-

12-07-2023 |
Team Kautilya Academy
-
.jpg)
12-06-2020 |
Kautilya Academy
-

12-05-2020 |
kautilya
-

12-05-2020 |
-

12-05-2020 |
-

12-05-2020 |
kauytilya
-
12-03-2020 |
Kautilya Academy
-

12-01-2019 |
Kautilya Academy
-

11-12-2019 |
Kautilya Academy
-

11-09-2023 |
Kautilya Academy
-

11-07-2020 |
Kautilya Academy
-

11-06-2020 |
Kautilya Academy
-

11-05-2021 |
Kautilya Academy
-

11-05-2020 |
Kautilya Academy
-

11-04-2023 |
Kautilya Academy
-

11-04-2023 |
Kautilya Academy
-

11-04-2019 |
Kautilya Academy
-

11-02-2020 |
Kautilya Academy
-

10-12-2020 |
Kautilya Academy
-

10-10-2022 |
Kautilya Academy
-

10-10-2020 |
Kautilya Academy
-

10-09-2020 |
Kautilya Academy
-

10-09-2020 |
Kautilya Academy
-

10-07-2020 |
Kautilya Academy
-

10-06-2020 |
Kautilya Academy
-

10-05-2021 |
Kautilya Academy
-

10-04-2020 |
Kautilya Academy
-

10-04-2019 |
Kautilya Academy
-

10-01-2020 |
Kautilya Academy
-
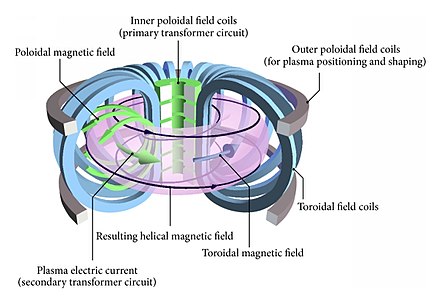
09-12-2020 |
Kautilya Academy
-

09-12-2019 |
Kautilya Academy
-

09-11-2020 |
Kautilya Academy
-

09-09-2020 |
Kautilya Academy
-

09-08-2023 |
Kautilya Academy
-

09-06-2020 |
Kautilya Academy
-

09-04-2020 |
Kautilya Academy
-

08-12-2020 |
Kautilya Academy
-
.jpg)
08-11-2019 |
Kautilya Academy
-

08-11-2019 |
Kautilya Academy
-
.jpg)
08-11-2019 |
Kautilya Academy
-

08-11-2019 |
Kautilya Academy
-

08-10-2020 |
-

08-09-2020 |
Kautilya Academy
-

08-07-2021 |
Kautilya Academy
-

08-06-2020 |
Kautilya Academy
-

08-05-2019 |
Kautilya Academy
-

08-05-2019 |
Kautilya Academy
-

08-04-2020 |
Kautilya Academy
-

08-01-2021 |
Kautilya Academy
-

08-01-2020 |
Kautilya Academy
-

07-12-2020 |
Kautilya Academy
-

07-12-2019 |
Kautilya Academy
-

07-11-2019 |
Kautilya Academy
-
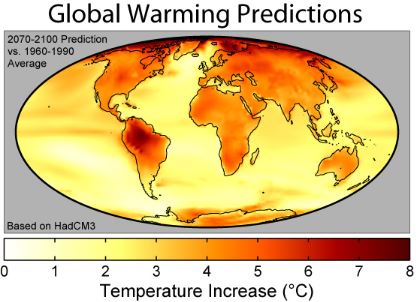
07-11-2019 |
Kautilya Academy
-

07-11-2019 |
Kautilya Academy
-

07-09-2020 |
Kautilya Academy
-

07-08-2019 |
Kautilya Academy
-

07-08-2019 |
KAUTILYA ACADEMY
-

07-08-2019 |
Kautilya academy
-

07-04-2020 |
Kautilya Academy
-

07-03-2020 |
Kautilya Academy
-

07-02-2020 |
Kautilya Academy
-
4.jpg)
07-01-2021 |
Kautilya Academy
-

07-01-2019 |
Kautilya Academy
-

06-12-2023 |
Kautilya Academy
-
1.jpg)
06-12-2019 |
Kautilya Academy
-

06-11-2020 |
-

06-11-2019 |
Kautilya Academy
-
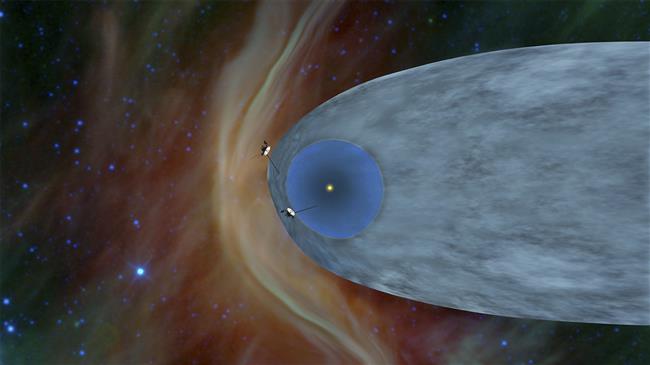
06-11-2019 |
Kautilya Academy
-

06-11-2019 |
Kautilya Academy
-

06-11-2019 |
Kautilya Academy
-

06-11-2019 |
Kautilya Academy
-
.jpg)
06-11-2019 |
Kautilya Academy
-

06-10-2020 |
Kautilya Academy
-

06-09-2019 |
Kautilya Academy
-

06-09-2019 |
Kautilya Academy
-

06-09-2019 |
Kautilya Academy
-

06-06-2020 |
Kautilya Academy
-

06-05-2020 |
Kautilya Academy
-

06-04-2020 |
Kautilya Academy
-

06-03-2023 |
Kautilya Academy
-

06-03-2023 |
Kautilya Academy
-

06-01-2023 |
Kautilya Academy
-
1.jpg)
06-01-2020 |
Kautilya Academy
-

05-12-2020 |
Kautilya Academy
-

05-10-2020 |
-

05-09-2020 |
Kautilya Academy
-

05-09-2020 |
Kautilya Academy
-
.jpg)
05-06-2021 |
Kautilya Academy
-

05-06-2020 |
Kautilya Academy
-

05-05-2020 |
Kautilya Academy
-

05-03-2020 |
Kautilya Academy
-

05-02-2020 |
Kautilya Academy
-

04-12-2020 |
Kautilya Academy
-

04-12-2019 |
kautilya academy
-

04-11-2020 |
Kautilya Academy
-

04-10-2023 |
Kautilya Academy
-

04-09-2020 |
Kautilya Academy
-
.jpg)
04-09-2019 |
Kautilya Academy
-

04-09-2019 |
Kautilya Academy
-

04-09-2019 |
Vishnugupta
-

04-09-2019 |
Kautilya Academy
-

04-09-2019 |
Kautilya Academy
-

04-06-2020 |
Kautilya Academy
-

04-05-2020 |
-

04-04-2020 |
Kautilya Academy
-
.jpg)
04-01-2024 |
Kautilya Academy
-

04-01-2020 |
Kautilya academy
-

03-12-2020 |
Kautilya Academy
-
4.jpg)
03-12-2019 |
Kautilya Academy
-

03-12-2019 |
Kautilya Academy
-

03-12-2019 |
Kautilya Academy
-
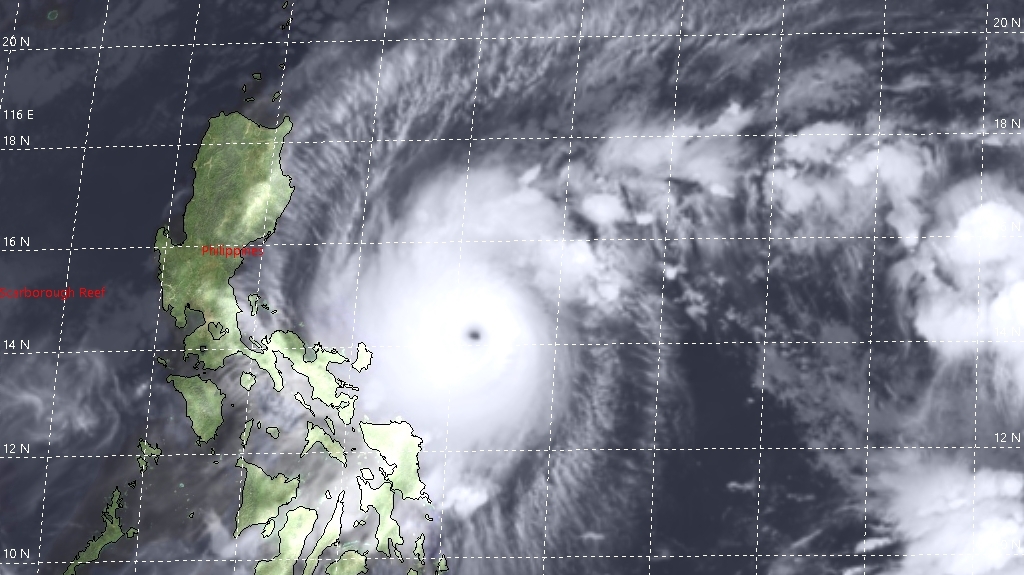
03-11-2020 |
-

03-10-2020 |
Kautilya Academy
-

03-09-2020 |
Kautilya Academy
-

03-08-2023 |
Kautilya Academy
-

03-08-2023 |
Kautilya Academy
-

03-06-2020 |
-

03-04-2020 |
Kautilya Academy
-

03-04-2019 |
Kautilya Academy
-

03-02-2020 |
Kautilya Academy
-

03-02-2020 |
Kautilya Academy
-

02-12-2020 |
Kautilya Academy
-

02-12-2020 |
Kautilya Academy
-

02-11-2020 |
Kautilya Academy
-

02-09-2020 |
Kautilya Academy
-

02-08-2019 |
Kautilya academy
-

02-07-2020 |
Kautilya Academy
-

02-06-2021 |
Kautilya Academy
-

02-06-2020 |
Kautilya Academy
-

02-05-2020 |
Kautilya Academy
-

02-03-2020 |
Kautilya Academy
-

02-02-2019 |
Kautilya Academy
-
.jpg)
02-02-2019 |
Kautilya Academy
-

02-02-2019 |
Kautilya Academy
-

01-09-2020 |
Kautilya Academy
-

01-08-2023 |
Kautilya Academy
-

01-07-2020 |
Kautilya Academy
-

01-06-2020 |
Kautilya Academy
-

01-05-2020 |
-

01-04-2020 |
Kautilya Academy





